Molecules and Crystals
Problem 6.191
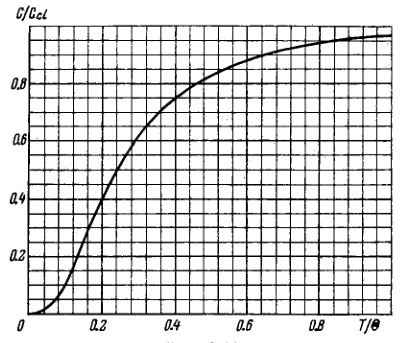
Fig. 6.10 shows heat capacity of a crystal vs temperature in terms of the Debye theory. Here \(C_{c l}\) is classical heat capacity, \(\Theta\) is the Debye temperature. Using this plot, find: (a) the Debye temperature for silver if at a temperature \(T=65 \mathrm{~K}\) its molar heat capacity is equal to \(15 \mathrm{~J} /(\mathrm{mol} \cdot \mathrm{K})\) (b) the molar heat capacity of aluminium at \(T=80 \mathrm{~K}\) if at \(T=250 \mathrm{~K}\) it is equal to \(22.4 \mathrm{~J} /(\mathrm{mol} \cdot \mathrm{K})\) (c) the maximum vibration frequency for copper whose heat capacity at \(T=125 \mathrm{~K}\) differs from the classical value by \(25 \%\).